Category Archive: Application Notes
Abstract
Introduction
In the relapsing remitting type of multiple sclerosis (MS) reducing relapses and neurodegeneration is crucial in halting the long-term impact of the disease. Medical disease-modifying treatments have proven effective, especially when introduced early in the disease course. However, patients still experience disease activity and disability progression, and therefore, supplemental early treatment strategies are warranted. Exercise appear to be one of the most promising supplemental treatment strategies, but a somewhat overlooked ‘window of opportunity’ exist early in the disease course. The objective of this study is to investigate exercise as a supplementary treatment strategy early in the disease course of MS.

Methods and analysis
The presented Early Multiple Sclerosis Exercise Study is a 48-week (plus 1-year follow-up) national multicentre single-blinded parallel group randomised controlled trial comparing two groups receiving usual care plus supervised high-intense exercise or plus health education (active control). Additionally, data will be compared with a population-based control group receiving usual care only obtained from the Danish MS Registry. The primary outcomes are annual relapse rate and MRI derived global brain atrophy. The secondary outcomes are disability progression, physical and cognitive function, MS-related symptoms, and exploratory MRI outcomes. All analyses will be performed as intention to treat.
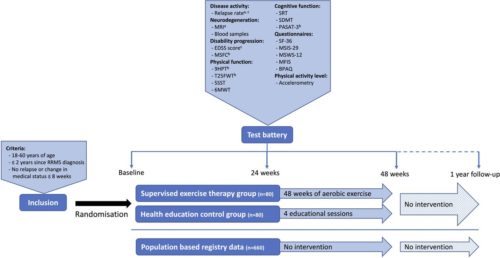
Figure 1 a: Primary outcome, b: Part of the Multiple Sclerosis Functional Composite, c: Outcomes available in the population based registry database.
Participants
Recruitment and eligibility
Patients with MS will be recruited via six Danish regional MS clinics (Aarhus University Hospital, Odense University Hospital, Clinics of Southern Denmark (Sønderborg, Esbjerg, Kolding), and Hospital Unit of Western Denmark), or via social media groups and events related to the Danish MS Society. In all cases, patients will be supplied with a leaflet explaining the rationale, design and content of the study and inviting them to participate. Those interested in participation will receive detailed written information as well as a leaflet from the National ethical committee explaining their rights as a participant in a health science research project. Furthermore, the project coordinator will contact participants by phone to explain the study, give the opportunity to ask questions, and to screen according to the inclusion criteria. Specifically, patients have to fulfil the following: (1) 18–60 years of age, (2) ≤2 years since clinical diagnosis with relapsing remitting MS and (3) no relapses or changes in medication ≤8 weeks prior to inclusion. Patients will be excluded if they: (1) are pregnant, or (2) have comorbidities or other issues thought to hinder participation in high intensity exercise activities. Finally, project nurses from the regional MS clinics confirm eligibility based on the patients’ medical records, and patients sign informed consent (standard formula from the Danish National Research Ethics Committee, see online supplemental material before inclusion.
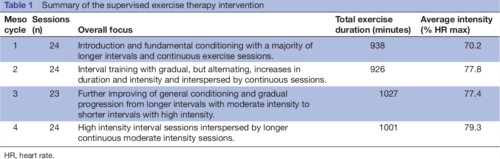 Table 1
Table 1 Summary of the supervised exercise therapy intervention
Primary outcomes
The overall primary outcomes of the study are annual relapse rate and MRI-derived annual global brain atrophy rate.
Relapse rate
The number of relapses in the study period is obtained from the medical records of the included patients. This will be done in collaboration with the respective MS clinics, and all relapses will be confirmed by a neurologist. According to the Danish national neurological association, a relapse is defined as new symptoms or worsening of existing symptoms, causing neurological dysfunction for a minimum of 24 hours without any signs of infection or fever. This exacerbation must be preceded by a stable 4-week period. The annual relapse rate is calculated for each patient by dividing 365 days with the number of days in the study and multiplying by the number of relapses in the study period. Due to the clinically meaningful definition of this outcome any potential changes in the relapse rate will be considered clinically meaningful.
Secondary outcomes
Disability progression
Neurological impairments in MS can be assessed by grading the impairment of eight different functional systems (pyramidal, cerebellar, brain stem, sensory, bowel and bladder, visual, cerebral, other) to rate the total EDSS score. The EDSS score is considered the gold standard when assessing disability and disease progression in MS and it is used routinely as a clinical endpoint in trials of DMTs. In this study, the EDSS score will be determined for each participant by trained neurologists at the six collaborative MS clinics during routine clinical visits. Therefore, the EDSS score nearest to the date of inclusion, to the date of completion, and to the date of 1 year since completion will be used to assess the progression of disabilities. However, recent studies have pointed out limitations in the EDSS score such as insufficient inter-rater and intrarater reproducibility and a low responsiveness—especially at the lower end of the scale. Therefore, the main measure of disability progression will be the Multiple Sclerosis Functional Composite (MSFC).
Inflammatory and neurodegenerative biomarkers
Blood samples will be collected in resting state at baseline, after 24 weeks, after 48 weeks, and again at 1-year follow-up. Patients will be allowed a minimum of 5 min supine rest before blood collection from the antecubital vein. Blood samples will be collected in ethylenediaminetetraacetic acid-treated tubes, resting for 90 min, and subsequently centrifuged at 1200 g for 10 min. Thereafter serum will be extracted and divided into five aliquots and stored at −80°C until further analyses.
Analysis of blood samples will be exploratory and aim to investigate the effects of exercise on relevant biomarkers, such as Glial Fibrillar Acid Protein, proinflammatory and anti-inflammatory markers (eg, interleukin-6, interleukin-10, interleukin-17, tumour necrosis factor-α) as well as neurodegenerative (eg, neurofilament light chain) and neurotrophic markers (eg, brain-derived neurotrophic factor, insulin-like growth factor).
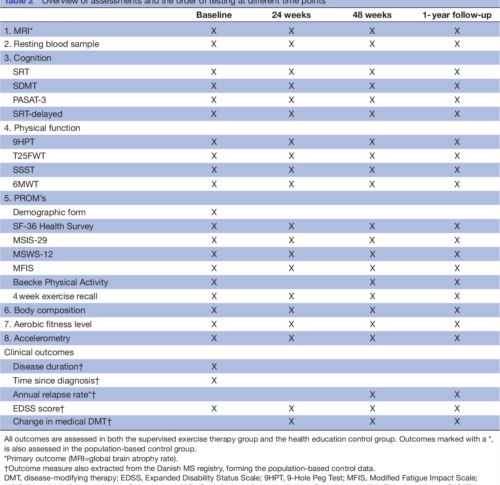
Table 2 Overview of assessments and the order of testing at different time points
DISCUSSION AND PERSPECTIVES
The presented study seeks to investigate supervised exercise as a supplemental treatment strategy early in the disease course of relapsing remitting MS. Effects of the
intervention will primarily be investigated on measures of disease activity and neurodegeneration, and secondarily on measures of disability progression, cognitive and physical function, and symptoms of fatigue. Efforts investigating the effects of exercise beyond rehabilitation (eg, as a supplemental disease-modifying strategy) are warranted and an overlooked ‘window of opportunity’ early in the disease course for MS exercise therapy have previously been identified. Importantly, the present study addresses both of these issues making the approach novel and innovative. Furthermore, the present study complies with the recommendations from
recent publications guiding the field of MS rehabilitation and exercise aligning the methodology with the current stage of the literature. Specifically, the study has
clearly defined primary outcomes and sample size calculations based hereupon (resulting in a large-scale exercise study), includes a rather long-term supervised exercise intervention and a well-monitored active control group (eg, importantly controlling the physical activity level of participants).
In summary, this is the first-ever study to investigate the effects of exercise in the very early stages of MS and thereby taking a more preventive approach aiming at lowering the disease activity more than medical DMTs alone aiming at maintaining (or even improving) functional and neurological reserve capacity. We expect the present study to hold the potential to change the current clinical practice regarding exercise therapy with MS. In particular, the present study may provide the first data supporting a warranted shift of paradigm where exercise will be considered a supplemental treatment strategy from an early timepoint in the disease course of MS. If these early exercise efforts show additional disease-modifying and neuroprotective effects, this is inherently of major interest to the individual MS patient, yet also to the healthcare system. While medical DMTs constitute the majority of healthcare costs for patients with mild MS early exercise efforts
may be a cost-effective supplemental treatment strategy to minimise disability progression and the huge-related costs. Another highly important perspective of early
exercise efforts as a supplemental treatment strategy in MS is the improvement of general health of the patients and the derived reduction in the increased risk of lifestyle-related comorbidities observed in patients with MS.


Authors: Morten Riemenschneider1, Lars G Hvid1, Steffen Ringgaard2, Mikkel K E Nygaard3, Simon F Eskildsen3, Thor Petersen4, Egon Stenager5,6, Ulrik Dalgas1
1 Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark
2 Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark.
3 The MR Research Centre, Aarhus University Hospital, Aarhus N, Denmark.
4 Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
5 The Multiple Sclerosis Clinic, Department of Neurology, Aarhus University Hospital, Aarhus, Denmark.
6 Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark.
 Published in: BMJ Open, 11 Jan 2021, 11(1):e043699
Published in: BMJ Open, 11 Jan 2021, 11(1):e043699
Abstract
Background: Oxygen is an essential therapy for hypoxemia but is scarce in low-income settings. Oxygen conserving devices optimize delivery, but to date have been designed for adults in high-income settings. Here we present the development and clinical pilot study of an oxygen-sparing nasal reservoir cannula (OSNRC) for pediatric use in low-income settings.

Methods
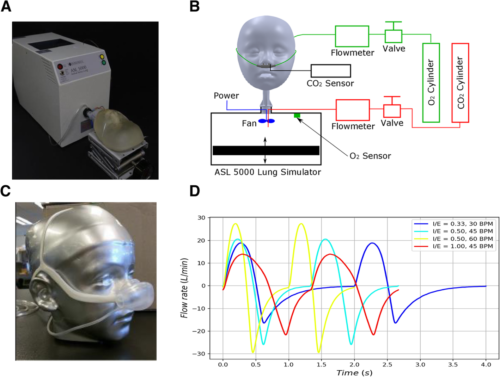
(1) Pre-clinical development of a novel OSNRC using a simulated respiratory circuit with metabolic simulator and anatomically accurate face-airway models. Simulated breathing waveforms were designed based on airway resistance, lung compliance, respiratory rate, and tidal volume of spontaneous breathing for three disease conditions.
(2) Pilot, randomized, controlled, non-blinded, cross-over study of the OSNRC vs standard nasal cannula (SNC) among children hospitalized with hypoxemic pneumonia in Uganda. Eight children were randomized to OSNRC followed by SNC, and eight were randomized to SNC followed by OSNRC.
Results
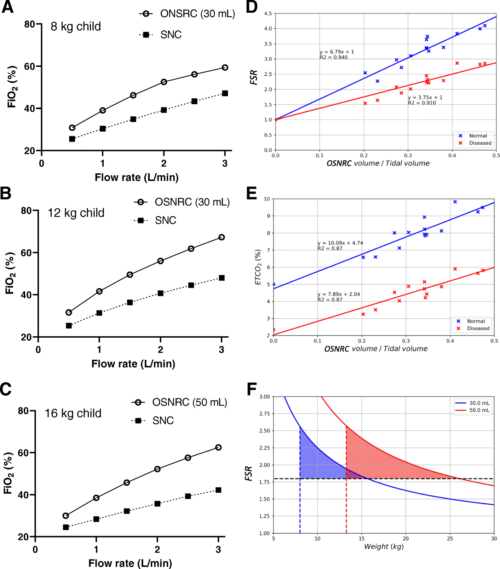
The laboratory simulation showed that the OSNRC provided the same or higher fraction of inspired oxygen at approximately 2.5-times lower flow rate compared to SNC. The flow savings ratio exhibited a linear relationship with the OSNRC volume to tidal volume ratio with a slope that varied with breathing waveforms. The range of performance from different breathing waveforms defined a performance envelope of the OSNRC. Two mask sizes (30 mL and 50 mL) provided sufficient coverage for patients between the 3rd and 97th percentile in our targeted age range. In the clinical pilot study, the rise in capillary blood pCO2 was similar in the OSNRC and SNC groups, suggesting that the OSNRC was not associated with CO2 retention. There were no significant differences between OSNRC and SNC with respect to clinical adverse events, lactate levels, pH, and SpO2. The OSNRC group had a higher mean SpO2 than the SNC group (adjusted mean difference, 1.4, 95% confidence interval 1.1 to 1.8), showing oxygen delivery enhancement.
Conclusion
The OSNRC enhances oxygen delivery without causing CO2 retention and appears to be well-tolerated by pediatric patients. If safety, efficacy and tolerability are confirmed in larger trials, this device has the potential to optimize oxygen delivery in children in low-resource settings, reducing the global burden of pediatric pneumonia.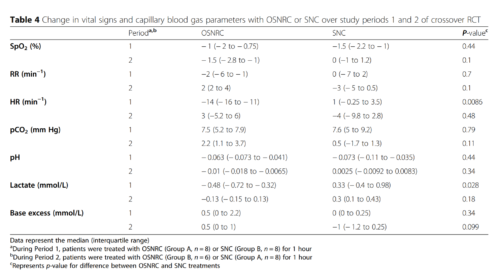
Authors: Jerry Mulond1 , Stella Maleni1, Hellen Aanyu-Tukamuhebwa2, Ezekiel Mupere2,3, Alfred Onubia Andama4, Chin Hei Ng5, Stephen Burkot5, Ella M. E. Forgie6, Qaasim Mian6, Christine M. Bachman5, Gerard Rummery7, Daniel Lieberman5, David Bell5,8, Michael T. Hawkes6,9,10,11,12*† and Akos Somoskovi5†
1 Infectious Diseases Research Collaboration, Kampala, Uganda.
2 Department of Pediatrics and Child Health, Mulago National Referral Hospital and Makerere University, Kampala, Uganda.
3 Department of Pediatrics, Makerere, University College of Health Sciences, Kampala, Uganda.
4 Department of Medicine, Makerere University College of Health Sciences, Kampala, Uganda.
5 Intellectual Ventures, Global Good Fund, Bellevue, WA, USA.
6 Department of Pediatrics, University of Alberta, 3-588D Edmonton Clinic Health Academy, 11405 87 Ave NW, Edmonton, Alberta T6G 1C9, Canada.
7 ResMed Ltd., Bella Vista, Australia.
8 Present address: Issaquah, USA.
9 Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, Canada.
10 School of Public Health, University of Alberta, Edmonton, Canada.
11 Stollery Science Lab, Edmonton, Canada.
12 Women and Children’s Health Research Institute, Edmonton, Canada
* Correspondence: mthawkes@ualberta.ca †
Michael T. Hawkes and Akos Somoskovi contributed equally to this work


Article published in: BMC Pulmonary Medicine volume 20, Article number: 230 (2020)

Efficacy and safety of oxygen-sparing nasal reservoir cannula for treatment of pediatric hypoxemic pneumonia in Uganda: a pilot randomized clinical trial
Oxigraf is proud of supporting the Thornhill Medical COVID-19 Response Action Plan!
Thornhill Medical is answering the call to support the fight against COVID-19, producing and delivering the MOVES® SLC™ devices to help save lives across the country. Combining an O₂ concentrator, ventilator, suction & vital signs monitoring in a rugged battery-powered portable unit, MOVES® SLC™ is a proven, mobile integrated life support technology. It’s Canadian advanced innovation at its best.
Watch Thornhill Medical in action:
https://thornhillmedical.com/en/news/dec-17-2020-thornhill-medical-covid-19-response-in-action/
Abstract
Reductions in prefrontal oxygenation near maximal exertion may limit exercise performance by impairing executive functions that influence the decision to stop exercising; however, whether deoxygenation also occurs in motor regions that more directly affect central motor drive is unknown. Multichannel near-infrared spectroscopy was used to compare changes in prefrontal, premotor, and motor cortices during exhaustive exercise. Twenty-three subjects performed two sequential, incremental cycle tests (25 W/min ramp) during acute hypoxia [79 Torr inspired Po(2) (Pi(O(2)))] and normoxia (117 Torr Pi(O(2))) in an environmental chamber. Test order was balanced, and subjects were blinded to chamber pressure. In normoxia, bilateral prefrontal oxygenation was maintained during low- and moderate-intensity exercise but dropped 9.0 +/- 10.7% (mean +/- SD, P < 0.05) before exhaustion (maximal power = 305 +/- 52 W). The pattern and magnitude of deoxygenation were similar in prefrontal, premotor, and motor regions (R(2) > 0.94). In hypoxia, prefrontal oxygenation was reduced 11.1 +/- 14.3% at rest (P < 0.01) and fell another 26.5 +/- 19.5% (P < 0.01) at exhaustion (maximal power = 256 +/- 38 W, P < 0.01). Correlations between regions were high (R(2) > 0.61), but deoxygenation was greater in prefrontal than premotor and motor regions (P < 0.05). Prefrontal, premotor, and motor cortex deoxygenation during high-intensity exercise may contribute to an integrative decision to stop exercise. The accelerated rate of cortical deoxygenation in hypoxia may hasten this effect.

Methods
Subjects.
After approval from the Colorado Multiple Institutional Review Board, 25 active, healthy volunteers (23 men and 2 women) from the Denver, CO, metropolitan area (elevation 1,650 m) provided written, informed consent to participate in a larger study investigating the etiology of acute mountain sickness. Physical examinations, including blood and urine tests, were conducted to verify general health before participation. All study procedures followed ethical guidelines established by the Declaration of Helsinki.
Study design.
Two incremental exercise tests were performed in an environmental chamber under ambient normobaric [normoxic (Norm), 610 Torr barometric pressure (Pb), 118 Torr inspired Po2 (PiO2)] and hypobaric [hypoxic (Hypox), 425 Torr Pb, 79 Torr PiO2] conditions to assess aerobic fitness for the larger study. Both tests were performed during a single chamber session to allow direct comparisons between Norm and Hypox without introduction of error from sensor placement/replacement. Tests were counterbalanced to control for order using a blinding strategy that varied chamber pressure to elicit similar sounds and changes in ear pressure during standardized 15-min ascent and descent periods. After arrival at the target Pb, 15 min were needed to adjust the cycle ergometer (Velotron Dynafit Pro, Racermate, Seattle, WA) and equip subjects with instrumental sensors (see below). Resting data were collected for 2 min before a 5-min warm-up at 50 W. Work rate was then incrementally increased using a 25 W/min ramp protocol to exhaustion. Subjects were blinded to elapsed time, power output, pedal revolutions per minute, and all physiological signals. Cool-down exercise was performed at 50 W for 5 min before chamber pressure was adjusted. After 15 min of ascent/decent and 15 min of rest at the second Pb, the protocol was repeated.
Results
Subjects.
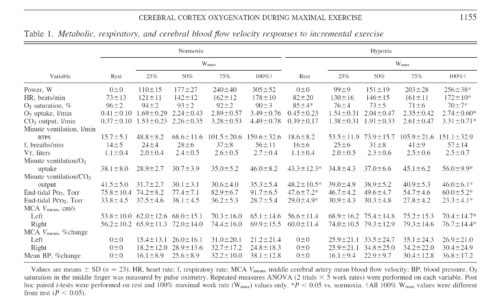
Twenty-three subjects (29 ± 8 yr of age, 73.7 ± 10.0 kg body wt, 181.7 ± 8.0 cm) completed both trials. Two subjects (with NIRS configuration 1) completed the Norm trial but were excluded from further study because of nausea and/or paresthesia during the subsequent depressurization period. Metabolic and power responses to both conditions were representative of physically fit, age-matched individuals (Table 1). Hypox reduced maximal V̇o2 and Wmax by 16 ± 6% and 21 ± 12%, respectively (P < 0.01). Order of trials did not affect the difference in maximal V̇o2 (P = 0.20) or Wmax (P = 0.95) between conditions, and 8 of 23 subjects were unable to retrospectively identify the order of testing.
Conclusions.
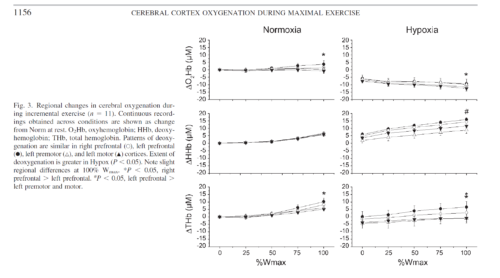
Cortical deoxygenation during high-intensity exercise is not restricted to prefrontal regions of the brain. Deoxygenation in premotor and motor cortices may contribute to fatigue and/or decisions to stop exercising. Acute hypoxia exacerbates cortical deoxygenation and, thus, may hasten these effects. Future functional NIRS studies are needed to expand our understanding of the role of cerebral activity in exhaustive whole body exercise.
Authors: Andrew W. Subudhi, Brittany R. Miramon, Matthew E. Granger, and Robert C. Roach
University of Colorado Altitude Research Center, Denver and Colorado Springs Campuses, Colorado Springs

Article published in: Journal of Applied Physiology 106: 1153–1158, 2009

Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia (pdf)
Quantum Computer Helium Laboratory
Oxygen Deficiency Monitor O2iM from Oxigraf:

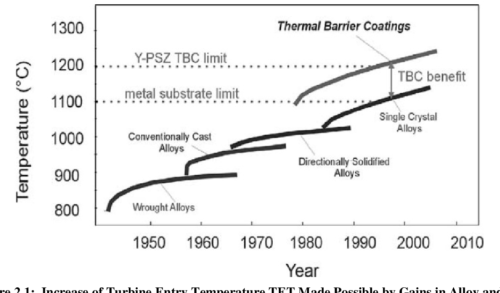
Turbine engine blades are subjected to extreme conditions characterized by significant and simultaneous excursions in both stress and temperature. These conditions promote thermo-mechanical fatigue (TMF) crack growth which can significantly reduce component design life beyond that which would be predicted from isothermal/constant load amplitude results. A thorough understanding of the thermo-mechanical fatigue crack behavior in single crystal superalloys is crucial to accurately evaluate component life to ensure reliable operations without blade fracture through the use of “retirement for cause” (RFC). This research was conducted on PWA1484, a single crystal superalloy used by Pratt & Whitney for turbine blades.


Initially, an isothermal constant amplitude fatigue crack growth rate database was developed, filling a void that currently exists in published literature. Through additional experimental testing, fractography, and modeling, the effects of temperature interactions, load interactions, oxidation and secondary crystallographic orientation on the fatigue crack growth rate and the underlying mechanisms responsible were determined. As is typical in published literature, an R Ratio of 0.7 displays faster crack growth when compared to R = 0.1. The effect of temperature on crack growth rate becomes more pronounced as the crack driving force increases. In addition secondary orientation and R Ratio effects on crack growth rate were shown to increase with increasing temperature. Temperature interaction testing between 649°C and 982°C showed that for both R = 0.1 and 0.7, retardation is present at larger alternating cycle blocks and acceleration is present at smaller alternating cycle blocks. This transition from acceleration to retardation occurs between 10 and 20 alternating cycles for R = 0.1 and around 20 alternating cycles for R = 0.7. Load interaction testing showed that when the crack driving force is near KIC the overload size greatly influences whether acceleration or retardation will occur at 982°C. Semi-realistic spectrum testing demonstrated the extreme sensitivity that relative loading levels play on fatigue crack growth life while also calling into question the importance of dwell times. A crack trajectory modeling approach using blade primary and secondary orientations was used to determine whether crack propagation will occur on crystallographic planes or normal to the applied load.
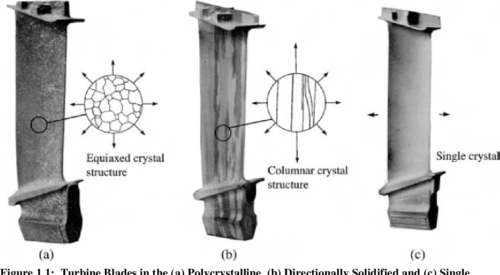
Crack plane determination using a scanning electron microscope enabled verification of the crack trajectory modeling approach. The isothermal constant amplitude fatigue crack growth results fills a much needed void in currently available data. While the temperature and load interaction fatigue crack growth results reveal the acceleration and retardation that is present in cracks growing in single crystal turbine blade materials under TMF conditions. This research also provides a deeper understanding of the failure and deformation mechanisms responsible for crack growth during thermo-mechanical fatigue. The crack path trajectory modeling will help enable “Retirement for Cause” to be used for critical turbine engine components, a drastic improvement over the standard “safe-life” calculations while also reducing the risk of catastrophic failure due to “chunk liberation” as a function of time. Leveraging off this work there exists the possibility of developing a “local approach” to define a crack growth forcing function in single crystal superalloys.
Author: Benjamin Scott Adair, Dissertation; George W. Woodruff School of Mechanical Engineering; Georgia Institute of Technology; August, 2013


Model O2iM – Oxygen Deficiency Safety Monitor:
The Oxigraf state of the art Oxygen Deficiency Monitor, the Model O2iM, is a fast response, accurate and reliable safety monitor for oxygen displacement monitoring in Quantum Computer Laboratory, MRI, NMR, and liquid nitrogen and helium storage facilities. Our reliable solid state sensor does not require routine maintenance or factory calibration, and the O2iM is equipped with an automatic/programmable auto-calibration system. The system easily interfaces with alarm system, HVAC controls, and building management systems.
Oxigraf Case Study:
State-of-the-art helium (and other rare gases) recovery, purification and liquefaction systems are required for operation of Helium-3/Helium-4 milli Kelvin dilution refrigerators in modern Quantum Computer Laboratories, liquid helium superconducting magnets (such as NMRs, MRIs, etc.), MEG systems for medical applications, cryogenic measurement cryostats, various size helium and cryogenic vacuum facilities.

The Problem:
Reliable solutions for sampling gas from remote locations in a Helium Processing Facility are needed in order to monitor equipment and personnel safety. During their operations, helium processing facilities are dealing with the presence of cryogenic nitrogen and helium, which presents oxygen deficiency hazards. Oxygen deficiency in the workplace can lead to blackouts, cause falls, and present more serious health risks — some of which can be fatal. The Oxigraf expert’s team can be brought in to help eliminate the risk of oxygen depletion.
The Solution:
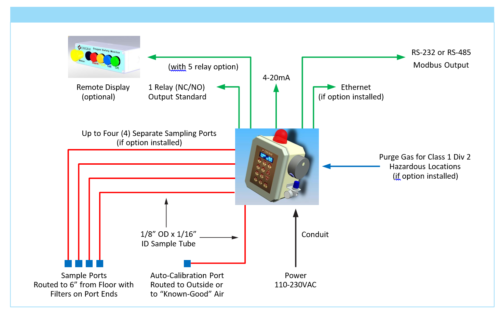
The Oxigraf Model O2iM, which has a high-flow pump option and allows for sampling from long distances. This sensor allows for continual monitoring of the clients’ facilities atmosphere from a safe location, and provides local alarms and interfaces with sophisticated safety features to prevent hazards such as cryogenic spills, which can lead to rapid displacement of breathing air.
Oxigraf’s top-of-the-line oxygen deficiency monitor is flexible and efficient, and provided the client with a reliable, immediate oxygen alarm for concentrations of less than 19.5%. It also eliminated the need for frequent recalibration or replacement of oxygen sensors, as well as the comprehensive, time-consuming maintenance often involved in sampling systems. The risk of false alarms and alarm failures can also be eliminated.


This unique sensor features a rapid response time of less than a second. The built-in pump draws gas remotely, allowing for these quick response times. In fact, we offer the best response speed/signal in the industry, and can add multiplexors (valving) in order to monitor four or more locations from over 100 feet away.The transit time of the gas sample through the sampling tube may be 1 second per meter of sampling tube with our standard pump or using our high flow option, a much faster response is possible on long tubing lengths. The high flow pump operates at a much faster rate and pre-fetches samples.
Additionally, this sensor is insensitive to movement, temperature and pressure changes, has auto-calibration for absolute accuracy, and includes options for multi-port and high-flow sampling. It also features a remote display and optional battery backup to allow for proper functioning during power interruptions. In addition, it can be fitted with a Z-Purge system, which allows the unit to be used in Class 1 Div 2 hazardous areas. The monitor includes a sampling pump, hydrophobic filter, and flow sensor, while the microprocessor controller maintains the flow at a constant value.

The Result:
When comparing the Oxigraf O2iM sensor to other O2 sensing solutions, it can be determined that O2iM is “the champion,” allowing for reliable performance 24/7. Oxigraf customers are particularly impressed with the unique engineering of the “Pre-Fetch” high-flow pump option, which allows for the monitoring of distant sample locations while maintaining fast response times.
Typical O2iM Installation:
Learn More:
Oxigraf has over 20 years of experience producing laser gas sensors and instruments, and is the leading manufacturer of laser absorption spectroscopy sensors for oxygen gas measurement and analysis. Oxigraf O2iM Oxygen Safety Monitors have been widely adapted in hundreds of facilities since 2004, replacing a wide range of less reliable electrochemical sensors. Oxigraf O2 and CO2 sensors, in particular, have been widely adapted by OEM manufacturers of medical respiratory gas monitors in order to measure breath waveforms, end-tidal gas values, anaerobic thresholds, VO2 maxs, and non-invasive cardiac outputs. For more information on our sensors, or to speak with an expert about your specific monitoring needs, contact the team today.
Please download your Oxigraf Case Study: Oxygen Monitor for Quantum Computer Helium Laboratory
BACKGROUND:
The accurate measurement of oxygen consumption (VO2) and energy expenditure (EE) may be helpful to optimize the treatment of critically ill patients. However, current techniques are limited in their ability to accurately quantify these end points in infants due to a low VO2, low tidal volume, and rapid respiratory rate. This study describes and validates a new device intended to perform in this size range.

METHODS:
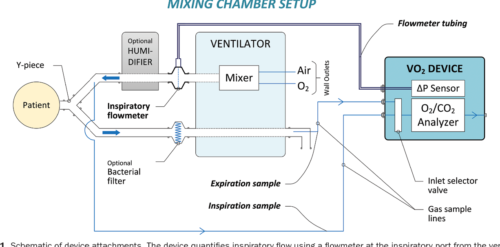
We created a customized device that quantifies inspiratory volume using a pneumotachometer and concentrations of oxygen and carbon dioxide gas in the inspiratory and expiratory limbs. We created a customized algorithm to achieve precise time alignment of these measures, incorporating bias flow and compliance factors. The device was validated in 3 ways. First, we infused a certified gas mixture (50% oxygen/50% carbon dioxide) into an artificial lung circuit, comparing measured with simulated VO2 and carbon dioxide production (VCO2) within a matrix of varying tidal volume (4–20 mL), respiratory rate (20–80 bpm), and fraction of inspired oxygen (0.21–0.8). Second, VO2, VCO2, and EE were measured in Sprague Dawley rats under mechanical ventilation and were compared to simultaneous Douglas bag collections. Third, the device was studied on n = 14 intubated, spontaneously breathing neonates and infants, comparing measured values to Douglas measurements. In all cases, we assessed for difference between the device and reference standard by linear regression and Bland–Altman analysis.
RESULTS:
In vitro, the mean ± standard deviation difference between the measured and reference standard VO2 was +0.04 ± 1.10 (95% limits of agreement, −2.11 to +2.20) mL/min and VCO2 was +0.26 ± 0.31 (−0.36 to +0.89) mL/min; differences were similar at each respiratory rate and tidal volume measured, but higher at fraction of inspired oxygen of 0.8 than at 0.7 or lower. In rodents, the mean difference was −0.20 ± 0.55 (−1.28 to +0.89) mL/min for VO2, +0.16 ± 0.25 (−0.32 to +0.65) mL/min for VCO2, and −0.84 ± 3.29 (−7.30 to +5.61) kcal/d for EE. In infants, the mean VO2 was 9.0 ± 2.5 mL/kg/min by Douglas method and was accurately measured by the device (bias, +0.22 ± 0.87 [−1.49 to +1.93] mL/kg/min). The average VCO2 was 8.1 ± 2.3 mL/kg/min, and the device exhibited a bias of +0.33 ± 0.82 (−1.27 to +1.94) mL/kg/min. Mean bias was +2.56% ± 11.60% of the reading for VO2 and +4.25% ± 11.20% of the reading for VCO2; among 56 replicates, 6 measurements fell outside of the 20% error range, and no patient had >1 of 4 replicates with a >20% error in either VO2 or VCO2.
CONCLUSIONS:
This device can measure VO2, VCO2, and EE with sufficient accuracy for clinical decision-making within the neonatal and pediatric size range, including in the setting of tachypnea or hyperoxia.
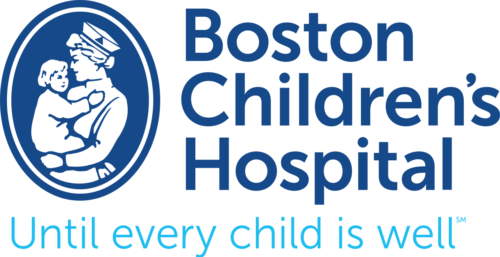
Authors: Nachman, Einav BS*; Clemensen, Peter MS*,†; Santos, Katheryn BS*; Cole, Alexis R. BS*; Polizzotti, Brian D. PhD*,‡; Hofmann, Grace RRT§; Leeman, Kristen T. MD‡,∥; van den Bosch, Sarah J. MS*; Kheir, John N. MD*,‡
*Department of Cardiology, Boston Children’s Hospital, Boston, Massachusetts
†Department of Research and Development, InnoCC, Glamsbjerg, Denmark
‡Department of Pediatrics, Harvard Medical School, Boston, Massachusetts
Departments of §Respiratory Care
∥Newborn Medicine, Boston Children’s Hospital, Boston, Massachusetts
Abstract
BACKGROUND:
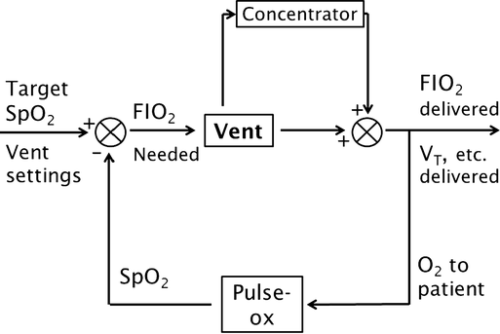
Addition of an oxygen concentrator into a control loop furthers previous work in autonomous control of oxygenation. Software integrates concentrator and ventilator function from a single control point, ensuring maximum efficiency by placing a pulse of oxygen at the beginning of the breath. We sought to verify this system. Methods: In a test lung, fraction of inspired oxygen (FIO2) levels and additional data were monitored. Tests were run across a range of clinically relevant ventilator settings in volume control mode, for both continuous flow and pulse dose flow oxygenation. Results: Results showed the oxygen concentrator could maintain maximum pulse output (192 mL) up to 16 breaths per minute. Functionality was verified across ranges of tidal volumes and respiratory rates, with and without positive end-expiratory pressure, in continuous flow and pulse dose modes. For a representative test at respiratory rate 16 breaths per minute, tidal volume 550 mL, without positive end-expiratory pressure, pulse dose oxygenation delivered peak FIO2 of 76.83 ± 1.41%, and continuous flow 47.81 ± 0.08%; pulse dose flow provided a higher FIO2 at all tested setting combinations compared to continuous flow (p < 0.001). Conclusions: These tests verify a system that provides closed loop control of oxygenation while integrating time-coordinated pulse-doses from an oxygen concentrator. This allows the most efficient use of resources in austere environments.

METHODS:
In a test lung, fraction of inspired oxygen (FIO2) levels and additional data were monitored. Tests were run across a range of clinically relevant ventilator settings in volume control mode, for both continuous flow and pulse dose flow oxygenation.
RESULTS:
Results showed the oxygen concentrator could maintain maximum pulse output (192 mL) up to 16 breaths per minute. Functionality was verified across ranges of tidal volumes and respiratory rates, with and without positive end-expiratory pressure, in continuous flow and pulse dose modes. For a representative test at respiratory rate 16 breaths per minute, tidal volume 550 mL, without positive end-expiratory pressure, pulse dose oxygenation delivered peak FIO2 of 76.83 ± 1.41%, and continuous flow 47.81 ± 0.08%; pulse dose flow provided a higher FIO2 at all tested setting combinations compared to continuous flow (p < 0.001).
CONCLUSIONS:
These tests verify a system that provides closed loop control of oxygenation while integrating time-coordinated pulse-doses from an oxygen concentrator. This allows the most efficient use of resources in austere environments.
. 

Authors: Thomas C. Blakeman (a) , Jay A. Johannigman (a,b), Matthew M. Gangidine (a,b), Richard D. Branson (a)
(a) University of Cincinnati Department of Surgery, Division of Trauma/Critical Care; Cincinnati, OH, United States
(b) Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814.

Published: Military Medicine, Volume 181, Issue suppl_5, 1 May 2016, Pages 177–183,
Abstract
Purpose
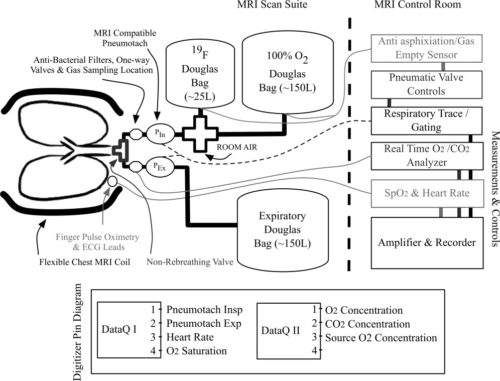
To evaluate the use of a modular MRI conditional respiratory monitoring and gating solution, designed to facilitate proper monitoring of subjects’ vital signals and their respiratory efforts, during free‐breathing and breathheld 19F, oxygen‐enhanced, and Fourier‐decomposition MRI‐based acquisitions.
Materials and Methods
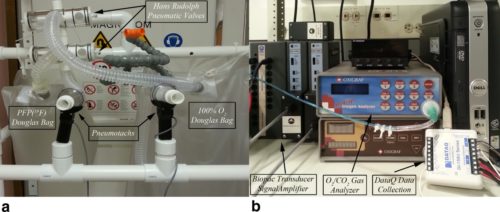
All Imaging was performed on a Siemens TIM Trio 3 Tesla MRI scanner, following Institutional Review Board approval. Gas delivery is accomplished through the use of an MR compatible pneumotachometer, in conjunction with two three‐way pneumatically controlled Hans Rudolph Valves. The pneumatic valves are connected to Douglas bags used as the gas source. A mouthpiece (+nose clip) or an oro‐nasal Hans Rudolph disposable mask is connected following the pneumatic valve to minimize dead‐space and provide an airtight seal. Continuous monitoring/sampling of inspiratory and expiratory oxygen and carbon dioxide levels at the mouthpiece/mask is achieved through the use of an Oxigraf gas analyzer.

Results
Forty‐four imaging sessions were successfully monitored, during Fourier‐decomposition (n = 3), fluorine‐enhanced (n = 29), oxygen‐enhanced, and ultra short echo (n = 12) acquisitions. The collected waveforms, facilitated proper monitoring and coaching of the subjects.
Conclusion
We demonstrate an inexpensive, off‐the‐shelf solution for monitoring these signals, facilitating assessments of lung function. Monitoring of respiratory efforts and exhaled gas concentrations assists in understanding the heterogeneity of lung function visualized by gas imaging. J. Magn. Reson. Imaging 2014;39:735–741. © 2013 Wiley Periodicals, Inc.

Authors: Ahmed F. Halaweish, PhD and H. Cecil Charles, PhD: Duke Image Analysis Laboratory and Department of Radiology, Duke University School of Medicine, Durham, North Carolina, USA,

Physiorack: An integrated MRI safe/conditional, Gas delivery, respiratory gating, and subject monitoring solution for structural and functional assessments of pulmonary function (pdf)