Study protocol: randomised controlled trial evaluating exercise therapy as a supplemental treatment strategy in early multiple sclerosis the Early Multiple Sclerosis Exercise Study (EMSES)
Abstract
Introduction
In the relapsing remitting type of multiple sclerosis (MS) reducing relapses and neurodegeneration is crucial in halting the long-term impact of the disease. Medical disease-modifying treatments have proven effective, especially when introduced early in the disease course. However, patients still experience disease activity and disability progression, and therefore, supplemental early treatment strategies are warranted. Exercise appear to be one of the most promising supplemental treatment strategies, but a somewhat overlooked ‘window of opportunity’ exist early in the disease course. The objective of this study is to investigate exercise as a supplementary treatment strategy early in the disease course of MS.
Methods and analysis
The presented Early Multiple Sclerosis Exercise Study is a 48-week (plus 1-year follow-up) national multicentre single-blinded parallel group randomised controlled trial comparing two groups receiving usual care plus supervised high-intense exercise or plus health education (active control). Additionally, data will be compared with a population-based control group receiving usual care only obtained from the Danish MS Registry. The primary outcomes are annual relapse rate and MRI derived global brain atrophy. The secondary outcomes are disability progression, physical and cognitive function, MS-related symptoms, and exploratory MRI outcomes. All analyses will be performed as intention to treat.
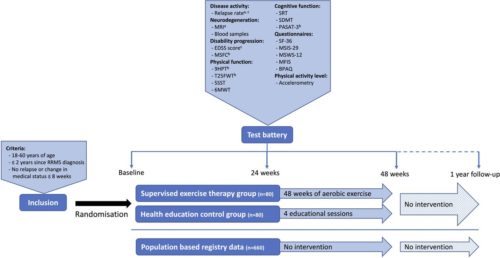
Figure 1 a: Primary outcome, b: Part of the Multiple Sclerosis Functional Composite, c: Outcomes available in the population based registry database.
Participants
Recruitment and eligibility
Patients with MS will be recruited via six Danish regional MS clinics (Aarhus University Hospital, Odense University Hospital, Clinics of Southern Denmark (Sønderborg, Esbjerg, Kolding), and Hospital Unit of Western Denmark), or via social media groups and events related to the Danish MS Society. In all cases, patients will be supplied with a leaflet explaining the rationale, design and content of the study and inviting them to participate. Those interested in participation will receive detailed written information as well as a leaflet from the National ethical committee explaining their rights as a participant in a health science research project. Furthermore, the project coordinator will contact participants by phone to explain the study, give the opportunity to ask questions, and to screen according to the inclusion criteria. Specifically, patients have to fulfil the following: (1) 18–60 years of age, (2) ≤2 years since clinical diagnosis with relapsing remitting MS and (3) no relapses or changes in medication ≤8 weeks prior to inclusion. Patients will be excluded if they: (1) are pregnant, or (2) have comorbidities or other issues thought to hinder participation in high intensity exercise activities. Finally, project nurses from the regional MS clinics confirm eligibility based on the patients’ medical records, and patients sign informed consent (standard formula from the Danish National Research Ethics Committee, see online supplemental material before inclusion.
Primary outcomes
The overall primary outcomes of the study are annual relapse rate and MRI-derived annual global brain atrophy rate.
Relapse rate
The number of relapses in the study period is obtained from the medical records of the included patients. This will be done in collaboration with the respective MS clinics, and all relapses will be confirmed by a neurologist. According to the Danish national neurological association, a relapse is defined as new symptoms or worsening of existing symptoms, causing neurological dysfunction for a minimum of 24 hours without any signs of infection or fever. This exacerbation must be preceded by a stable 4-week period. The annual relapse rate is calculated for each patient by dividing 365 days with the number of days in the study and multiplying by the number of relapses in the study period. Due to the clinically meaningful definition of this outcome any potential changes in the relapse rate will be considered clinically meaningful.
Secondary outcomes
Disability progression
Neurological impairments in MS can be assessed by grading the impairment of eight different functional systems (pyramidal, cerebellar, brain stem, sensory, bowel and bladder, visual, cerebral, other) to rate the total EDSS score. The EDSS score is considered the gold standard when assessing disability and disease progression in MS and it is used routinely as a clinical endpoint in trials of DMTs. In this study, the EDSS score will be determined for each participant by trained neurologists at the six collaborative MS clinics during routine clinical visits. Therefore, the EDSS score nearest to the date of inclusion, to the date of completion, and to the date of 1 year since completion will be used to assess the progression of disabilities. However, recent studies have pointed out limitations in the EDSS score such as insufficient inter-rater and intrarater reproducibility and a low responsiveness—especially at the lower end of the scale. Therefore, the main measure of disability progression will be the Multiple Sclerosis Functional Composite (MSFC).
Inflammatory and neurodegenerative biomarkers
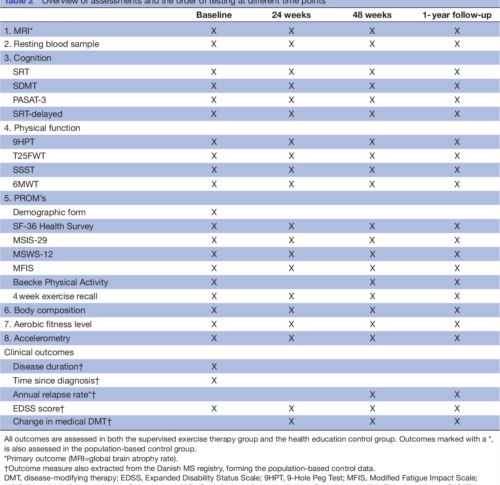
Blood samples will be collected in resting state at baseline, after 24 weeks, after 48 weeks, and again at 1-year follow-up. Patients will be allowed a minimum of 5 min supine rest before blood collection from the antecubital vein. Blood samples will be collected in ethylenediaminetetraacetic acid-treated tubes, resting for 90 min, and subsequently centrifuged at 1200 g for 10 min. Thereafter serum will be extracted and divided into five aliquots and stored at −80°C until further analyses.
Analysis of blood samples will be exploratory and aim to investigate the effects of exercise on relevant biomarkers, such as Glial Fibrillar Acid Protein, proinflammatory and anti-inflammatory markers (eg, interleukin-6, interleukin-10, interleukin-17, tumour necrosis factor-α) as well as neurodegenerative (eg, neurofilament light chain) and neurotrophic markers (eg, brain-derived neurotrophic factor, insulin-like growth factor).
DISCUSSION AND PERSPECTIVES
The presented study seeks to investigate supervised exercise as a supplemental treatment strategy early in the disease course of relapsing remitting MS. Effects of the
intervention will primarily be investigated on measures of disease activity and neurodegeneration, and secondarily on measures of disability progression, cognitive and physical function, and symptoms of fatigue. Efforts investigating the effects of exercise beyond rehabilitation (eg, as a supplemental disease-modifying strategy) are warranted and an overlooked ‘window of opportunity’ early in the disease course for MS exercise therapy have previously been identified. Importantly, the present study addresses both of these issues making the approach novel and innovative. Furthermore, the present study complies with the recommendations from
recent publications guiding the field of MS rehabilitation and exercise aligning the methodology with the current stage of the literature. Specifically, the study has
clearly defined primary outcomes and sample size calculations based hereupon (resulting in a large-scale exercise study), includes a rather long-term supervised exercise intervention and a well-monitored active control group (eg, importantly controlling the physical activity level of participants).
In summary, this is the first-ever study to investigate the effects of exercise in the very early stages of MS and thereby taking a more preventive approach aiming at lowering the disease activity more than medical DMTs alone aiming at maintaining (or even improving) functional and neurological reserve capacity. We expect the present study to hold the potential to change the current clinical practice regarding exercise therapy with MS. In particular, the present study may provide the first data supporting a warranted shift of paradigm where exercise will be considered a supplemental treatment strategy from an early timepoint in the disease course of MS. If these early exercise efforts show additional disease-modifying and neuroprotective effects, this is inherently of major interest to the individual MS patient, yet also to the healthcare system. While medical DMTs constitute the majority of healthcare costs for patients with mild MS early exercise efforts
may be a cost-effective supplemental treatment strategy to minimise disability progression and the huge-related costs. Another highly important perspective of early
exercise efforts as a supplemental treatment strategy in MS is the improvement of general health of the patients and the derived reduction in the increased risk of lifestyle-related comorbidities observed in patients with MS.
Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark
Exercise Biology, Department of Public Health, Aarhus University, Aarhus, Denmark.
3 The MR Research Centre, Aarhus University Hospital, Aarhus N, Denmark.
4 Center of Functionally Integrative Neuroscience, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
5 The Multiple Sclerosis Clinic, Department of Neurology, Aarhus University Hospital, Aarhus, Denmark.
6 Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark.