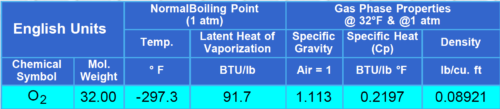
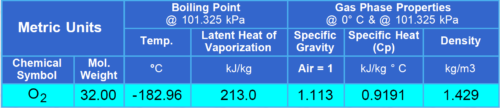
Properties of Oxygen
Oxygen makes up 20.94% by volume or 23% by weight of the air we breathe. It is colorless, odorless and tasteless. It is the most common element on earth. It forms compounds with virtually all chemical elements except the noble gases, and most oxygen is thus bound with other elements in compounds such as silicates, oxides, and water. It is also dissolved in rivers, lakes, and oceans. Almost all molecular oxygen occurs in the atmosphere.
Oxygen is highly oxidizing (a general chemical term applying to any substance, like oxygen, that accepts electrons from another substance during reaction). Oxygen reacts vigorously with combustible materials, especially in its pure state, generating heat in the reaction process.
Oxygen is the second-largest volume industrial gas. It is used in steelmaking and other metals refining and fabrication processes, in chemicals, pharmaceuticals, petroleum processing, glass and ceramic manufacture, pulp and paper manufacture, healthcare, environmental protection through treatment of municipal and industrial effluents.