Abstract
Background: Oxygen is an essential therapy for hypoxemia but is scarce in low-income settings. Oxygen conserving devices optimize delivery, but to date have been designed for adults in high-income settings. Here we present the development and clinical pilot study of an oxygen-sparing nasal reservoir cannula (OSNRC) for pediatric use in low-income settings.
Methods
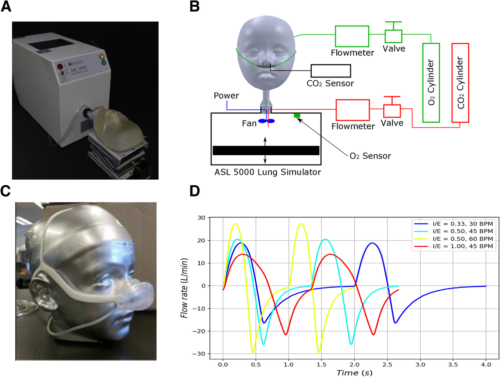
(1) Pre-clinical development of a novel OSNRC using a simulated respiratory circuit with metabolic simulator and anatomically accurate face-airway models. Simulated breathing waveforms were designed based on airway resistance, lung compliance, respiratory rate, and tidal volume of spontaneous breathing for three disease conditions.
(2) Pilot, randomized, controlled, non-blinded, cross-over study of the OSNRC vs standard nasal cannula (SNC) among children hospitalized with hypoxemic pneumonia in Uganda. Eight children were randomized to OSNRC followed by SNC, and eight were randomized to SNC followed by OSNRC.
Results
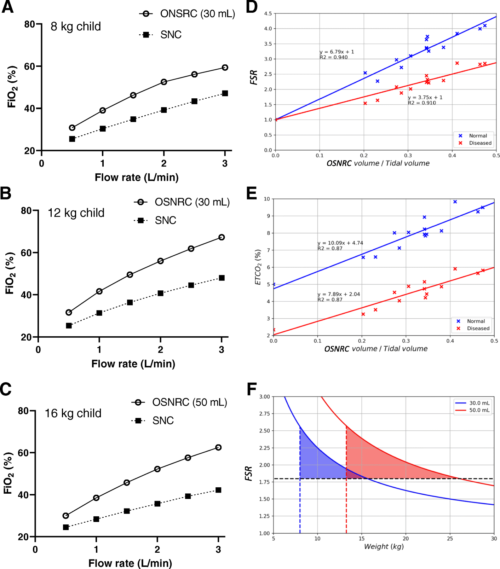
The laboratory simulation showed that the OSNRC provided the same or higher fraction of inspired oxygen at approximately 2.5-times lower flow rate compared to SNC. The flow savings ratio exhibited a linear relationship with the OSNRC volume to tidal volume ratio with a slope that varied with breathing waveforms. The range of performance from different breathing waveforms defined a performance envelope of the OSNRC. Two mask sizes (30 mL and 50 mL) provided sufficient coverage for patients between the 3rd and 97th percentile in our targeted age range. In the clinical pilot study, the rise in capillary blood pCO2 was similar in the OSNRC and SNC groups, suggesting that the OSNRC was not associated with CO2 retention. There were no significant differences between OSNRC and SNC with respect to clinical adverse events, lactate levels, pH, and SpO2. The OSNRC group had a higher mean SpO2 than the SNC group (adjusted mean difference, 1.4, 95% confidence interval 1.1 to 1.8), showing oxygen delivery enhancement.
Conclusion
The OSNRC enhances oxygen delivery without causing CO2 retention and appears to be well-tolerated by pediatric patients. If safety, efficacy and tolerability are confirmed in larger trials, this device has the potential to optimize oxygen delivery in children in low-resource settings, reducing the global burden of pediatric pneumonia.
Authors: Jerry Mulond1 , Stella Maleni1, Hellen Aanyu-Tukamuhebwa2, Ezekiel Mupere2,3, Alfred Onubia Andama4, Chin Hei Ng5, Stephen Burkot5, Ella M. E. Forgie6, Qaasim Mian6, Christine M. Bachman5, Gerard Rummery7, Daniel Lieberman5, David Bell5,8, Michael T. Hawkes6,9,10,11,12*† and Akos Somoskovi5†
1 Infectious Diseases Research Collaboration, Kampala, Uganda.
2 Department of Pediatrics and Child Health, Mulago National Referral Hospital and Makerere University, Kampala, Uganda.
3 Department of Pediatrics, Makerere, University College of Health Sciences, Kampala, Uganda.
4 Department of Medicine, Makerere University College of Health Sciences, Kampala, Uganda.
5 Intellectual Ventures, Global Good Fund, Bellevue, WA, USA.
6 Department of Pediatrics, University of Alberta, 3-588D Edmonton Clinic Health Academy, 11405 87 Ave NW, Edmonton, Alberta T6G 1C9, Canada.
7 ResMed Ltd., Bella Vista, Australia.
8 Present address: Issaquah, USA.
9 Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, Canada.
10 School of Public Health, University of Alberta, Edmonton, Canada.
11 Stollery Science Lab, Edmonton, Canada.
12 Women and Children’s Health Research Institute, Edmonton, Canada
* Correspondence: mthawkes@ualberta.ca †
Michael T. Hawkes and Akos Somoskovi contributed equally to this work
Article published in: BMC Pulmonary Medicine volume 20, Article number: 230 (2020)





COMMENTS