A Device for the Quantification of Oxygen Consumption and Caloric Expenditure in the Neonatal Range
Leave a CommentBACKGROUND:
The accurate measurement of oxygen consumption (VO2) and energy expenditure (EE) may be helpful to optimize the treatment of critically ill patients. However, current techniques are limited in their ability to accurately quantify these end points in infants due to a low VO2, low tidal volume, and rapid respiratory rate. This study describes and validates a new device intended to perform in this size range.
METHODS:
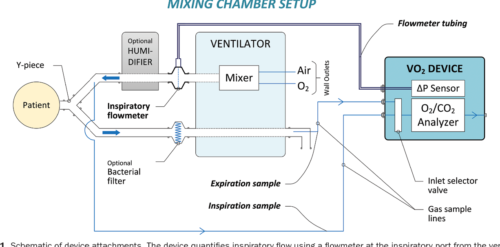
We created a customized device that quantifies inspiratory volume using a pneumotachometer and concentrations of oxygen and carbon dioxide gas in the inspiratory and expiratory limbs. We created a customized algorithm to achieve precise time alignment of these measures, incorporating bias flow and compliance factors. The device was validated in 3 ways. First, we infused a certified gas mixture (50% oxygen/50% carbon dioxide) into an artificial lung circuit, comparing measured with simulated VO2 and carbon dioxide production (VCO2) within a matrix of varying tidal volume (4–20 mL), respiratory rate (20–80 bpm), and fraction of inspired oxygen (0.21–0.8). Second, VO2, VCO2, and EE were measured in Sprague Dawley rats under mechanical ventilation and were compared to simultaneous Douglas bag collections. Third, the device was studied on n = 14 intubated, spontaneously breathing neonates and infants, comparing measured values to Douglas measurements. In all cases, we assessed for difference between the device and reference standard by linear regression and Bland–Altman analysis.
RESULTS:
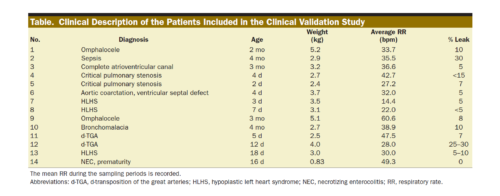
In vitro, the mean ± standard deviation difference between the measured and reference standard VO2 was +0.04 ± 1.10 (95% limits of agreement, −2.11 to +2.20) mL/min and VCO2 was +0.26 ± 0.31 (−0.36 to +0.89) mL/min; differences were similar at each respiratory rate and tidal volume measured, but higher at fraction of inspired oxygen of 0.8 than at 0.7 or lower. In rodents, the mean difference was −0.20 ± 0.55 (−1.28 to +0.89) mL/min for VO2, +0.16 ± 0.25 (−0.32 to +0.65) mL/min for VCO2, and −0.84 ± 3.29 (−7.30 to +5.61) kcal/d for EE. In infants, the mean VO2 was 9.0 ± 2.5 mL/kg/min by Douglas method and was accurately measured by the device (bias, +0.22 ± 0.87 [−1.49 to +1.93] mL/kg/min). The average VCO2 was 8.1 ± 2.3 mL/kg/min, and the device exhibited a bias of +0.33 ± 0.82 (−1.27 to +1.94) mL/kg/min. Mean bias was +2.56% ± 11.60% of the reading for VO2 and +4.25% ± 11.20% of the reading for VCO2; among 56 replicates, 6 measurements fell outside of the 20% error range, and no patient had >1 of 4 replicates with a >20% error in either VO2 or VCO2.
CONCLUSIONS:
This device can measure VO2, VCO2, and EE with sufficient accuracy for clinical decision-making within the neonatal and pediatric size range, including in the setting of tachypnea or hyperoxia.
Easter Greetings
Leave a CommentHappy Easter from all at Oxigraf!

World Health Day
Leave a CommentToday is World Health Day, and we want to say to every healthcare worker in the medical field who helps keep us healthy:
Thank you for everything you do, you are loved and appreciated!
System Design Verification for Closed Loop Control of Oxygenation With Concentrator Integration
Leave a CommentAbstract
BACKGROUND:
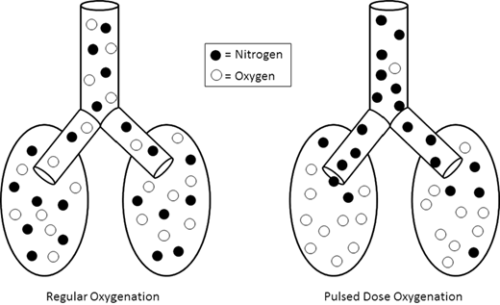
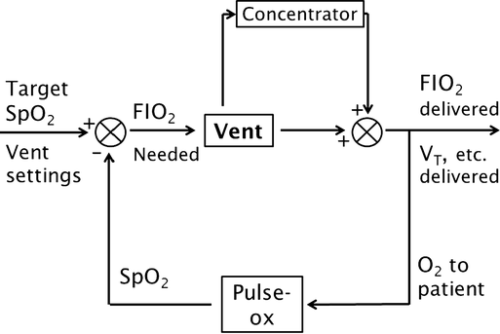
Addition of an oxygen concentrator into a control loop furthers previous work in autonomous control of oxygenation. Software integrates concentrator and ventilator function from a single control point, ensuring maximum efficiency by placing a pulse of oxygen at the beginning of the breath. We sought to verify this system. Methods: In a test lung, fraction of inspired oxygen (FIO2) levels and additional data were monitored. Tests were run across a range of clinically relevant ventilator settings in volume control mode, for both continuous flow and pulse dose flow oxygenation. Results: Results showed the oxygen concentrator could maintain maximum pulse output (192 mL) up to 16 breaths per minute. Functionality was verified across ranges of tidal volumes and respiratory rates, with and without positive end-expiratory pressure, in continuous flow and pulse dose modes. For a representative test at respiratory rate 16 breaths per minute, tidal volume 550 mL, without positive end-expiratory pressure, pulse dose oxygenation delivered peak FIO2 of 76.83 ± 1.41%, and continuous flow 47.81 ± 0.08%; pulse dose flow provided a higher FIO2 at all tested setting combinations compared to continuous flow (p < 0.001). Conclusions: These tests verify a system that provides closed loop control of oxygenation while integrating time-coordinated pulse-doses from an oxygen concentrator. This allows the most efficient use of resources in austere environments.
METHODS:
In a test lung, fraction of inspired oxygen (FIO2) levels and additional data were monitored. Tests were run across a range of clinically relevant ventilator settings in volume control mode, for both continuous flow and pulse dose flow oxygenation.
RESULTS:
Results showed the oxygen concentrator could maintain maximum pulse output (192 mL) up to 16 breaths per minute. Functionality was verified across ranges of tidal volumes and respiratory rates, with and without positive end-expiratory pressure, in continuous flow and pulse dose modes. For a representative test at respiratory rate 16 breaths per minute, tidal volume 550 mL, without positive end-expiratory pressure, pulse dose oxygenation delivered peak FIO2 of 76.83 ± 1.41%, and continuous flow 47.81 ± 0.08%; pulse dose flow provided a higher FIO2 at all tested setting combinations compared to continuous flow (p < 0.001).
CONCLUSIONS:
These tests verify a system that provides closed loop control of oxygenation while integrating time-coordinated pulse-doses from an oxygen concentrator. This allows the most efficient use of resources in austere environments.
Authors: Thomas C. Blakeman (a) , Jay A. Johannigman (a,b), Matthew M. Gangidine (a,b), Richard D. Branson (a)
(a) University of Cincinnati Department of Surgery, Division of Trauma/Critical Care; Cincinnati, OH, United States
(b) Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814.